Abstract
FCR001 is an investigational hematopoietic stem cell product manufactured to achieve a target dose of CD34+ cells, Facilitating cells (CD8+, TCR- cells), and a fixed number of αβ T cells. We previously demonstrated durable WBC and T cell donor chimerism in 26 of 37 living donor kidney recipients treated in a Phase 2 study utilizing non-myeloablative conditioning with Fludarabine (30 mg/kg) for 3 days, a single dose of Cyclophosphamide (50 mg/kg), and a single fraction of TBI (200 cGy) prior to infusion with FCR001 the day following a living donor kidney transplant from the same donor (median follow up 7.8 years, range 4-12). Donor cells were mobilized utilizing G-CSF, and the apheresis product was processed in a central GMP facility to manufacture the FCR001 product. Patients received a single dose of post-transplant Cyclophosphamide (50mg/kg) on day 2, and regimented tacrolimus and MMF. Immunosuppression was weaned after 6 months if there was donor T cell chimerism ≥50% with no evidence of GvHD, renal allograft biopsy-proven acute rejection (BPAR), or development of donor specific antibody. 23 of the initial cohort have remained off immunosuppression with stable renal graft function and donor T cell chimerism. Preliminary evaluation of immune reconstitution has previously been reported, with brisk neutrophil recovery, B and NK cell recovery within 6 months and T cell recovery within 12-18 months (Leventhal, J, et al, Transplantation, 2015).
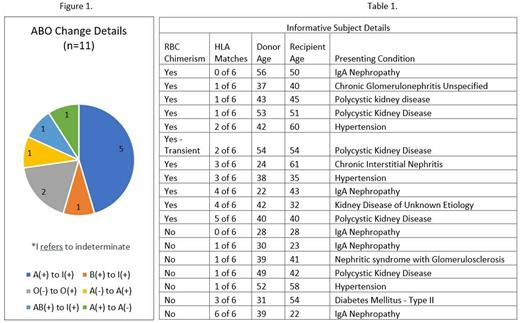
We sought to examine the potential for red blood cell (RBC) transplant indications by reviewing the results of RBC chimerism in this cohort. 18 of 37 patients had informative RBC discrepancies between donor and recipient, in either the ABO or Rh systems. 1 patient had transient WBC and RBC chimerism which reverted to the original recipient type at month 4 post-transplant. 56% (10/18) of patients showed evidence of long-term RBC chimerism as evidenced by a change in Rh or ABO type, or an indeterminate blood type with both donor and recipient typing reported (Fig 1). These patients (n=10) have all demonstrated stable, long term T cell chimerism at levels > 85% at a median of 8.5 years from transplant (range 4-12). All 10 have been weaned off all immunosuppression with no evidence of graft loss, or transplant related conditions including Evans syndrome, AIHA, TMA, transfusion dependence, or chronic GvHD. HLA matching in the RBC chimeric patients ranged from 0/6 to 5/6 (A, B, DR) and was not predictive of RBC chimerism in the entire subset of 18 patients in this analysis. Donor and recipient age, underlying indication for kidney transplant, and HLA matching were all evaluated and are shown in (Table 1).
This data shows that hematopoietic stem cell transplantation using FCR001 has the potential to establish durable RBC chimerism in patients with minimal requirements for HLA matching and a low incidence of chronic GvHD. This has therapeutic implications for the treatment of hemoglobinopathies and red cell disorders including sickle cell disease, thalassemia, and other genetic disorders involving RBC, where a chimeric state has the potential to ameliorate, or potentially cure, the underlying disease. Further modifications to the non-myeloablative conditioning regimen may allow for greater levels of RBC engraftment and chimerism while maintaining the minimal transplant related toxicity seen in this Phase 2 cohort. An ongoing, randomized Phase 3 trial (FREEDOM-1) has been initiated in the living donor kidney transplant population, and a Phase 2 trial (FREEDOM-3) has been initiated with FCR001, to explore its potential as a treatment for scleroderma. These studies will enable further analysis of the FCR001 stem cell product to achieve multi-lineage chimerism in additional patients, allowing further exploration of potential new indications in other acquired and genetic disease states amenable to stem cell transplantation.
Disclosures
Weinthal:Talaris Therapeutics: Current Employment, Current equity holder in publicly-traded company. Olsen:Talaris Therapeutics: Current Employment, Current equity holder in publicly-traded company. Schneider:Talaris Therapeutics: Current Employment, Current equity holder in private company. Krieger:Talaris Therapeutics, Inc: Current Employment, Current equity holder in publicly-traded company. Maakaron:Gilead: Research Funding; CRISPR Therapeutics: Research Funding; Precision BioSciences: Research Funding; Scripps: Research Funding; Fate Therapeutics: Research Funding; ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.